
Acid Base Reactions Worksheet
In a strong acid - strong base titration, neutralization produces water and an aqueous solution of a salt, whose cation and anion come from the base and acid, respectively. Neither ion is acidic or basic, so the pH at the equivalence point is that of neutral water; i.e., 7.00.

Acid and Base Worksheet 1 0708 ans key
Introductory Chemistry (CK-12) 21: Acids and Bases
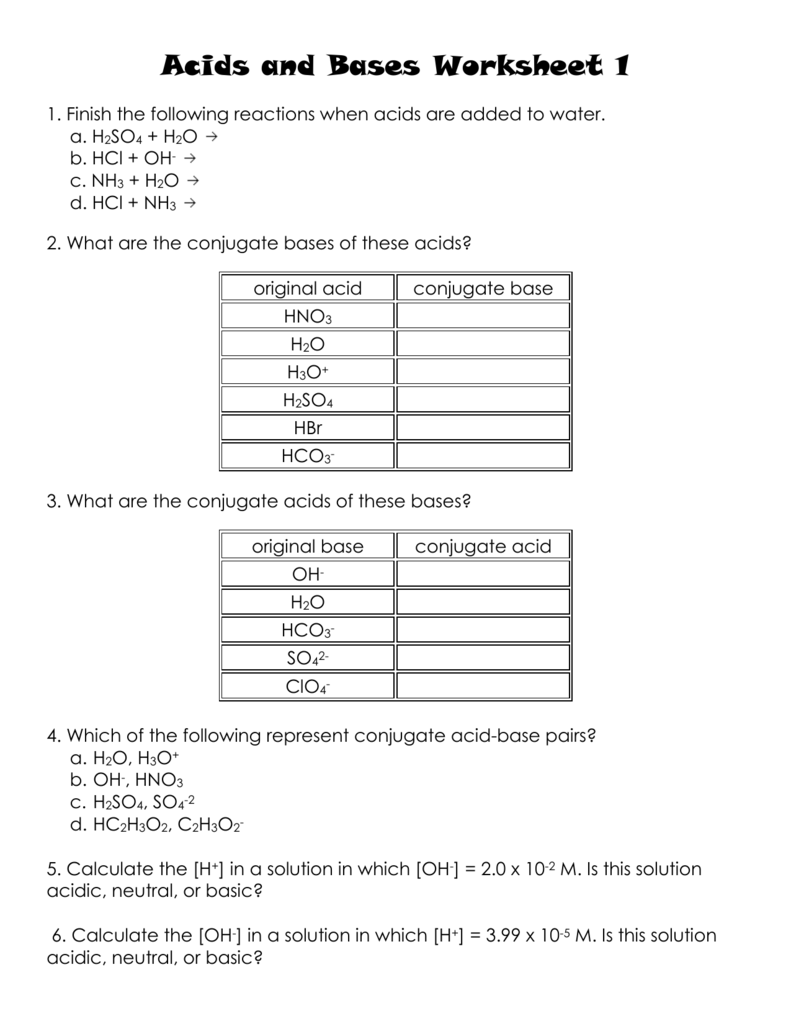
Acids and Bases Worksheet 1
1. For all the reactants and products, draw Lewis structures. 2. Identify the nucleophile (base) and electrophile (acid) in the reaction. 3. Draw curved arrows to show the flow of electrons. 4. Determine if the reaction can be termed a Brønsted-Lowry acid-base reaction. 7 PRACTICE PROBLEM

Phet Acids And Bases Worksheet Phet Acid Base Worksheets Teaching
Basic Basic pH + pOH = 14 14-.23 = 13.77 Date Name Acids & Bases Calculations Practice Worksheet Directions: Solve the followingpH calculations. Write the formula, plug numbers into formula, & give answer with correct units If the pH of a solution is 10.3, what is the [H+] concentration? -c M HC104, what is the pH?

Acids And Bases Calculations Practice Worksheet Answer Key
Acid and Base pH Calculations - Supplemental Worksheet KEY For each of the following solutions: Write a chemical equation, identify the limiting reactant (if there is one), and calculate the pH. We will calculate the pH of the solutions using the following 3 steps for each problem. Step 1: What is left in solution?
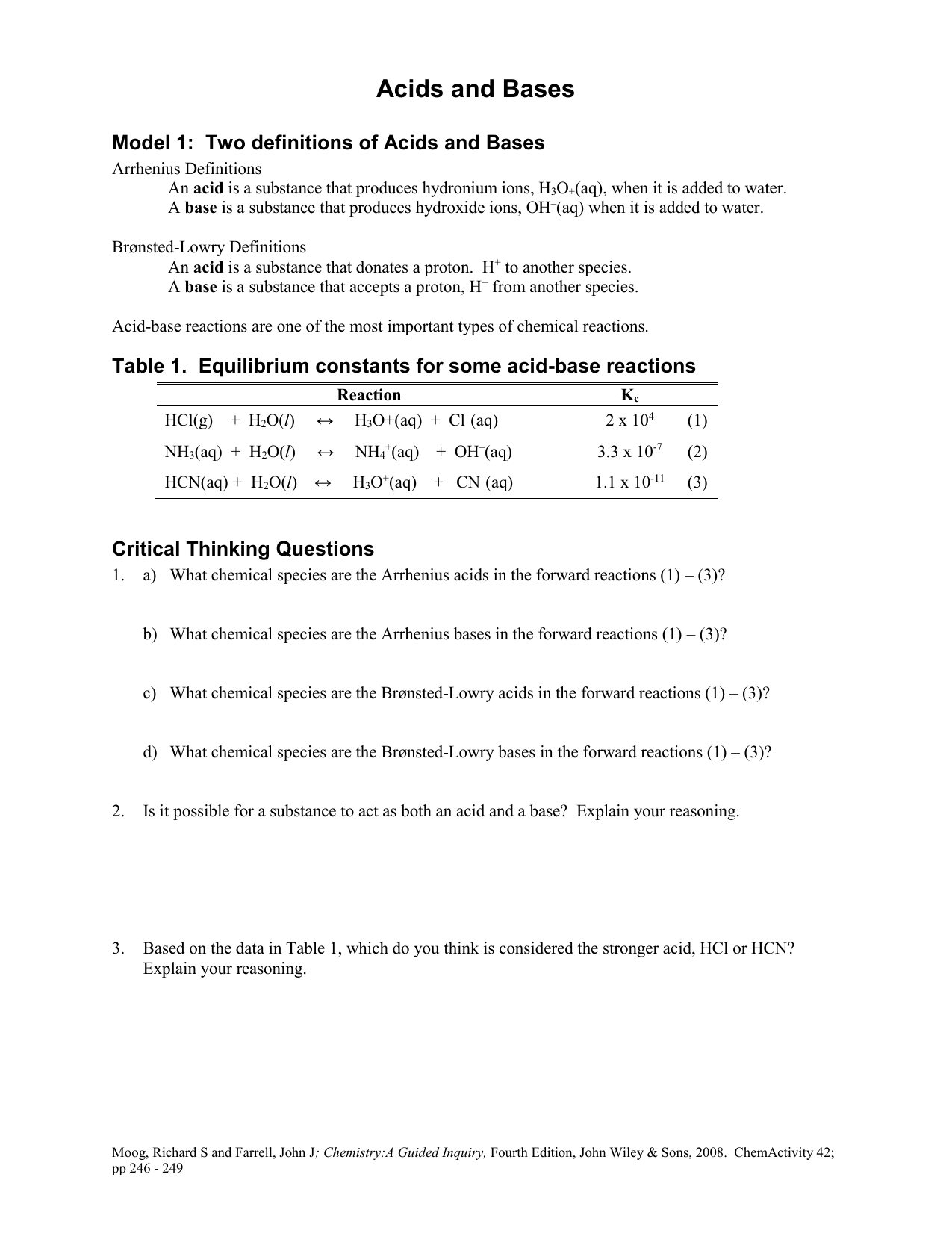
Acid Base Reaction Worksheet
This Bronsted Lowry acid base worksheet teaches how the hydrogen atoms move within an acid bases reaction. The student can look at the product and reactant sides of the reaction and see how the hydrogens are increasing or decreasing within a species.. example calculations and simple acid-base lab. Answers are included.Learning Objectives.
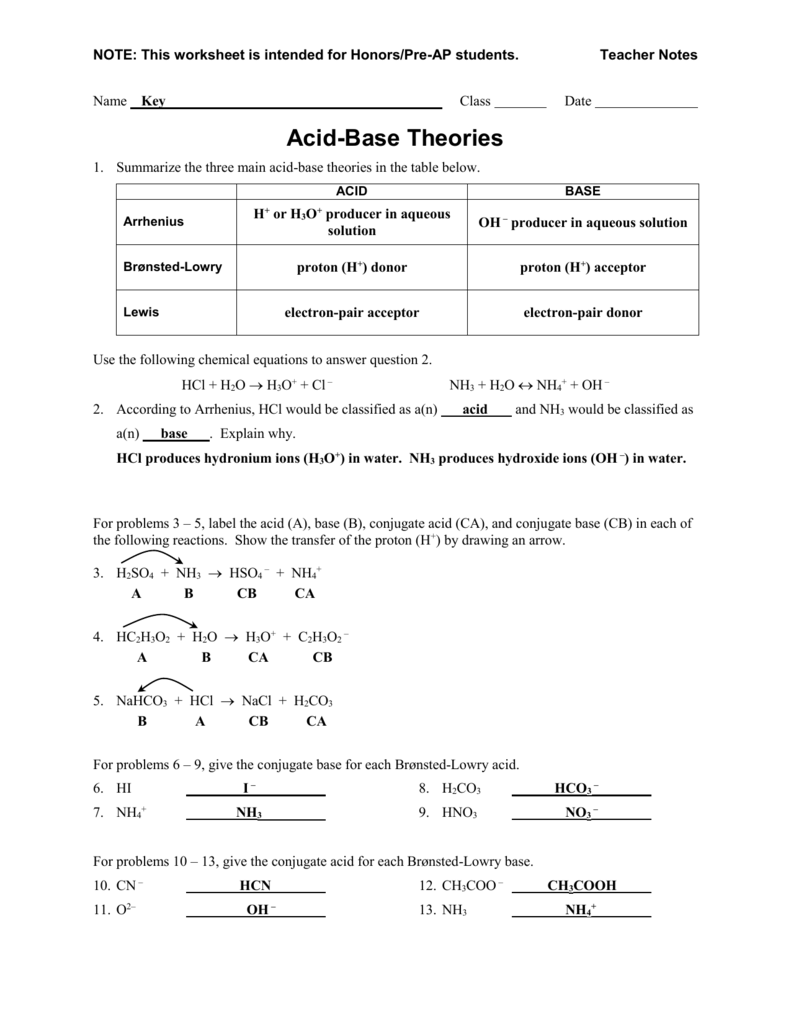
Acid And Base Worksheet
Given the solutions with the same initial concentration of each base, the higher percent of ionization is base A because it is the weakest base. 3. a. pKa + pKb = 14 → pKa = 14 − pKb = 14 − 3.80 = 10.2. Ka = 10 − pKa = 10 − 10.2 = 6.31 × 10 − 11. b. pKa + pKb = 14 → pKa = 14 − pKb = 14 − 7.90 = 6.10.

10++ Conjugate Acid Base Pairs Worksheet Worksheets Decoomo
ACIDS & BASES PRACTICE WORKSHEET 1. Without doing any calculations, identify each of the following solutions as acids or basic: a) [H 3 O +] = 3.4x10-8 M _____ b) [OH-. Identify the Bronsted-Lowry acid and base conjugate pairs for each of the following

Acids And Bases Calculations Practice Worksheet Answer Key
Conceptual Questions. Acids, Bases, and Conjugates, Miscellaneous 1. In the Brønsted-Lowry definition of acids and bases, an acid __________ a. is a proton donor. b. is a proton acceptor. c. forms stable hydrogen bonds. d. breaks stable hydrogen bonds. e. corrodes metals. 2. In the Brønsted-Lowry definition of acids and bases, a base __________
Acid Base pH and pKa Calculations in MCAT Chemistry Tutorial Video Series
Browse acid and bases calculations worksheet resources on Teachers Pay Teachers, a marketplace trusted by millions of teachers for original educational resources. Browse Catalog Grade Level Pre-K - K 1 - 2 3 - 5 6 - 8 9 - 12 Other Subject Arts & Music English Language Arts World Language Math Science Social Studies - History Specialty
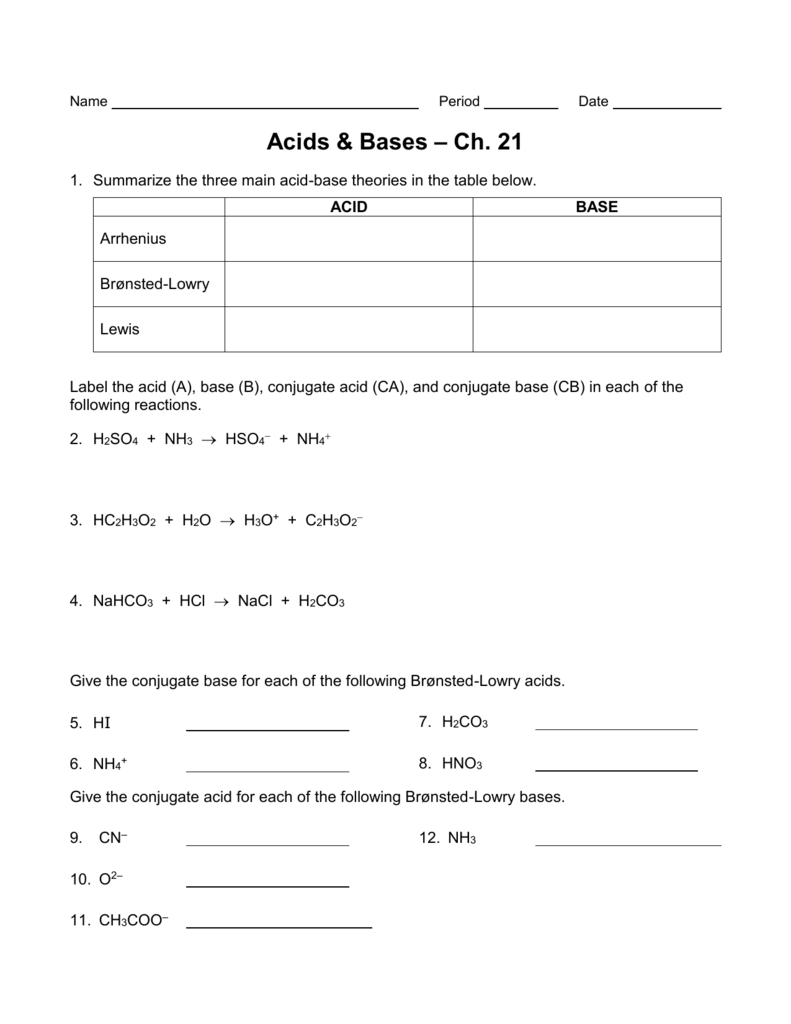
Acid And Base Worksheet
Acids/Bases & pH Worksheet (continued) Complete the following table by filling in the empty spaces. Indicate if the solution is acidic, basic or neutral. You should be able to do the starred (*) item s without a calculator. (Problem #23-34) [H 3O]
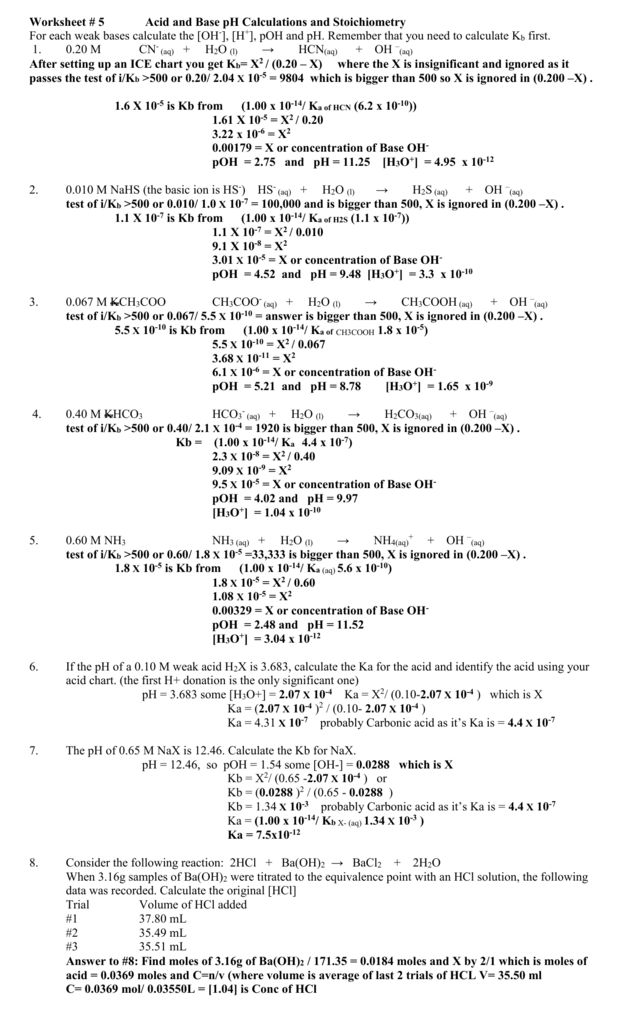
Worksheet 5 Acid and Base pH Calculations and Stoichiometry
Acids & Bases Calculations Practice Worksheet Directions: Solve the following pH calculations. Write the formula, plug numbers into formula, & give answer with. [OH-] pOH Acid / Base -1 x 10 3 M - 1 x 10 -8 M 6 2 -2.3 x 10 10 M -8.5 x 10 1 M -6.9 x 10 4 M -5.1 x 10 11 M . Author: Hughes, Brooke Created Date: 4/22/2015 2:25:31 PM.

Acids Bases and pH Scale Lab Activities Reading Comprehension
Acid Base - pH Calculations Name: Date: Period: Seat #: Show all work strong acid solution - determine [H+], calculate pH (2.903) Calculate the pH of 0.00125M HNO. WORKSHEET #1 . diprotic acid solution - assume all [H+] from first ionization, determine [H+] using ICE box, calculate pH. Calculate the pH of 0.00125M H 2CO 3 K

Teach your students about the pH scale and calculating pH of acids and
We have seen that the calculation of [H 3 O +] and pH for solutions of strong acids and base.To carry out a calculation of all species present in a solution of a pure weak acid in water requires use of the equilibrium constant for the acid's hydrolysis, called K a.In similar fashion, calculating the concentrations of all species in a solution of a weak base in water requires solving the.

AcidBase Info and Practice Problems
TASK 1 - Bronsted-Lowry acids & bases Identify the Bronsted-Lowry acid and base in each of the following reactions. SECTION 2 - pH of strong acids Number of protons released Monoprotic acid = acid that releases one H+ ion per molecule e.g. HCl (hydrochloric acid), HNO3 (nitric acid), CH3COOH (ethanoic acid) Diprotic acid =

Acids And Bases Worksheet
Brønsted-Lowry. The Brønsted-Lowry definition of acids and bases liberates the acid-base concept from its limitation to aqueous solutions, as well as the requirement that bases contain the hydroxyl group. A Brønsted-Lowry acid is a hydrogen-containing species which is capable of acting as a proton (hydrogen ion) donor. A Brønsted-Lowry base is a species which is capable of acting.